Cleaning Structures for Chemically Sensitized Individuals | Part 3
Part 3 - Neutralization

In the first part of this series, basic information was offered regarding the causes of chemical sensitization and the impact that such an experience has on a person’s life. I also warned readers that I was willing to “name names” of various products we have identified. It can be helpful in addressing situations where sensitized individuals are involved. I remind the readers again that we have no financial ties in the form of sales commissions, referral fees, etc. to any of the items referenced in this series.
The second installment focused on the importance of cleaning. Since chemical sensitization often involves minute quantities of materials which are invisible, resting on surfaces, and even the air inside the structure, scrupulous cleaning is generally the first step toward improving the environment for the impacted individuals. The general rule of thumb in these circumstances is if you cannot get the surfaces visibly clean, it is next to impossible to get them scientifically clean. This is a term that refers to measuring cleaned surfaces and evaluating the effectiveness of the work by determining how much of a particular residue is left.

What Is Neutralization?
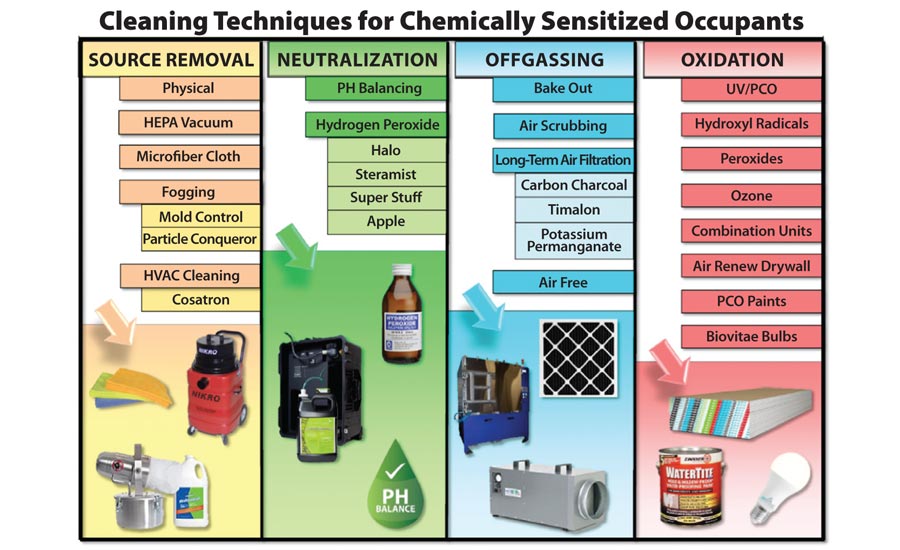
The Oxford dictionary provides four different definitions for the word “neutralize.”1 Two of those four definitions are useful in the discussion of how to properly deal with chemically sensitized individuals. The primary definition of the word neutralize refers to balancing the pH of different types of chemicals.
Chemically sensitized individuals may be working or living in buildings which have a residue that needs to be cleaned. We have often dealt with clients whose trigger event that caused the sensitization was the application of some product in the structure. Insect bombs, fuel oil spills, spray applied pesticides, carpet cleaning solutions, duct cleaning sprays, applications of chemicals “guaranteed” to kill mold, and even the overuse of candles and fragrances are some of the more common chemical situations that have brought clients to Wonder Makers. In such cases, identifying the pH level of the chemical residue is essential in providing the sensitized individual with an effective response plan.
Chemical neutralization improves the effectiveness of the cleaning process in two ways: 1) by applying acids to residue that are alkaline; 2) matching a cleaning product with an alkaline structure to surfaces that test acidic. Not only does the remaining material become less hazardous if it is properly neutralized, it also is often drawn out of the crevices on the surface, making it easier to remove. In contrast, trying to clean a surface by using a cleaner that is also on the lower half of the pH scale often results in the contamination being driven further into the surface on a microscopic basis.
Assisting the Neutralization Process in Removing Residue
Of course, when cleaning for chemical residues, all surfaces should be visibly clean. This may mean a detailed HEPA vacuuming of dry matter or the extraction of all puddled liquids is undertaken. At that point, the method used for applying cleaners when chemical residues are present can be just as important as selecting the cleaning agent that will have a pH neutralizing effect. Spray and wipe cleaning processes are not as effective in such situations as foam and wipe or foam and squeegee techniques.
One of the more interesting and useful technology transfers to the restoration industry over the past five years has been the growing acceptance of foam applicators in place of sprayers. Our favorite foam application units in regards to price and quality come from FOAM-iT. They have a wide variety of units that are as small as a 1 quart hand spray bottle (although in this case it really is a “foam bottle”) to a 30 gallon portable unit that can apply a thick swath of foam 20 feet up a wall. The 2 gallon foamer that looks like a garden sprayer is probably the most common in the industry today.
Another Definition for the Term Neutralize
In certain situations, the term neutralize can mean to kill or destroy. This aspect of the term also comes into play with chemically sensitized individuals. In some cases, chemical sensitization can be caused by byproducts from biological organisms such as: enzymes from bacteria or mycotoxins from mold. In such cases, the neutralization may include the destruction of the microorganisms on the surface as well as the chemical balancing of the offending substance.
In these cases, neutralization cleaning takes on a whole new meaning as it moves into the realm of disinfection. Although this may sound scary, there are many choices of cleaners and applicators for this type of neutralization. Two of the most common approaches to neutralization/disinfection are the proper application of an alcohol or hydrogen peroxide-based product.
Using alcohol as a neutralizing agent does not get as much attention in the United States as it does in other parts of the world. Some mold remediation professionals recommend a high proof grain alcohol (such as Everclear) mixed with water as a cleaning agent for the final step in a mold remediation process. Some individuals even recommend spraying such mixes, although the safety concerns regarding the flammability of such solutions are significant. That is why Biomist utilizes a system where highly concentrated alcohol can be sprayed safely because it is atomized and propelled with carbon dioxide rather than compressed air. Using the system for chemically- sensitized individuals rather than the traditional infection control projects have benefits because the alcohol vaporizes quickly and completely which allows the sensitized occupant back into the space after only a short period of time.
Understanding VHP
Hydrogen peroxide is another product that vaporizes quickly into non-hazardous byproducts and is well tolerated by chemically sensitized individuals. This technology, often referred to as vaporized hydrogen peroxide or VHP, has been migrating to the restoration field from the health care arena over the past decade. The two most well recognized systems in the restoration field are: 1) Steramist by TOMI Environmental Solutions; 2) Halo disinfection system by Halosil International.
Although both systems use hydrogen peroxide as a starting point, there are some real differences between the two. Steramist ionizes the material being sprayed which improves the coverage as the ionized droplets “wrap around” various surfaces. Halo utilizes a process described as a dry mist for their application. Obviously, there are a lot more details related to Biomist, Steramist, Halo, and other neutralization/disinfection systems available on the market. Any professional investigating these techniques to help them deal with chemically sensitized individuals (or mold-sensitized people) should use due diligence before they purchase; especially since these type of systems are fairly expensive.
Does Neutralization Work?
The quick and positive answer to the big question of whether any of these neutralization approaches work is the classic consultant response: “Yes, but....” Such procedures can be very effective when used as an adjunct to detailed cleaning rather than as a substitute. Many studies have concluded that neutralization does occur with the proper processes and products are matched against the problematic chemical or biological residue. However, the importance of the initial cleaning, as discussed in part two of this series, cannot be overstated.
Dealing with chemically sensitized clients can be a challenge for both a consultant and restoration contractor. Fortunately, the knowledge, products, and equipment are available so that real assistance can be offered to such suffering individuals.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








